✔ 100% Authentic Product
👁️ Currently Viewing 1458
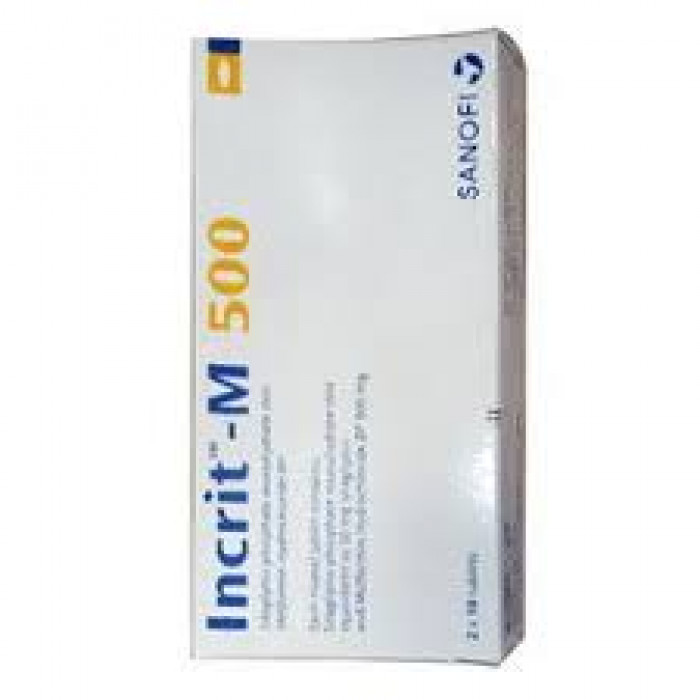
Incrit-M 500mg 10pcs
Tablet, Sitagliptin + Metformin Hydrochloride 50 mg+500 mg, Sanofi Bangladesh Limited
Discount
Price: ৳ 197
MRP:
৳
201
2%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Indications
When both sitagliptin and metformin therapy is suitable, this is suggested as an addition to diet and exercise to enhance glycemic control in individuals with type 2 diabetes mellitus. Important use restrictions:
This should not be used in individuals with type 1 diabetes or to treat diabetic ketoacidosis because it is ineffective in these cases.
This has not been examined in people who have had pancreatitis in the past. It's unclear whether individuals who have had pancreatitis in the past are more likely to develop pancreatitis while using This.
Pharmacology
This pill contains two antihyperglycemic medicines with complimentary modes of action to help patients with type 2 diabetes improve their glycemic control. Metformin HCl, a member of the biguanide family, and Sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor. Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that is thought to work in type 2 diabetes patients by delaying the inactivation of incretin hormones. The gut releases incretin hormones throughout the day, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), and levels rise in response to a meal.DPP-4, an enzyme, quickly inactivates these hormones. The incretins are part of an endogenous system that regulates glucose homeostasis in the body. GLP-1 and GIP enhance insulin production and release from pancreatic beta cells via intracellular signaling pathways including cyclic AMP when blood glucose levels are normal or high. GLP-1 also inhibits the release of glucagon by pancreatic alpha cells, resulting in decreased hepatic glucose synthesis. Sitagliptin enhances insulin release and lowers glucagon levels in the circulation in a glucose-dependent manner by raising and prolonging active incretin levels.Metformin HCl has a distinct pharmacologic mode of action than other oral antihyperglycemic drugs. Metformin HCl reduces hepatic glucose synthesis, lowers glucose absorption in the intestine, and improves glucose uptake and utilization in the peripheral tissues.
Dosage & Administration
Dose of film-coated tablet: The dosage of this tablet should be individualized on the basis of the patient's current regimen, efectiveness, and tolerability while not exceeding the maximum recommended daily dose of 100 mg sitagliptin and 2000 mg metformin. Initial combination therapy or maintenance of combination therapy should be individualized and left to the discretion of the health care provider.
This tablet should generally be given twice daily with meals, with gradual dose escalation, to reduce the gastrointestinal (GI) side efects due to metformin.
The starting dose of this tablet should be based on the patient’s current regimen. This tablet should be given twice daily with meals.
The recommended starting dose in patients not currently treated with metformin is 50 mg sitagliptin/500 mg metformin hydrochloride twice daily, with gradual dose escalation recommended to reduce gastrointestinal side efects associated with metformin.
The starting dose in patients already treated with metformin should provide sitagliptin dosed as 50 mg twice daily (100 mg total daily dose) and the dose of metformin already being taken. For patients taking metformin 850 mg twice daily, the recommended starting dose of this tablet is 50 mg sitagliptin/1000 mg metformin hydrochloride twice daily.
No studies have been performed specifcally examining the safety and efcacy of Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP in patients previously treated with other oral antihyperglycemic agents and switched to Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP. Any change in therapy of type 2 diabetes should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
Dose of extended-release tablet: Administer once daily with a meal preferably in the evening. Gradually escalate the dose to reduce the gastrointestinal side effects due to Metformin. May adjust the dosing based on effectiveness and tolerability while not exceeding the maximum recommended daily dose of 100 mg Sitagliptin and 2000 mg Metformin extended-release. Maintain the same total daily dose of Sitagliptin and Metformin when changing between film-coated tablet and extended-release tablet, without exceeding the maximum recommended daily dose of 2000 mg Metformin extended-release.
Patients using two extended-release tablets (such as two 50/500 or two 50/1000 tablets) should take the two tablets together once daily. The 100 mg Sitagliptin/1000 mg Metformin HCI extended-release tablet should be taken as a single tablet once daily.
Patients treated with an insulin secretagogue or insulin: Co-administration of the combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower doses of the insulin secretagogue or insulin to reduce the risk of hypoglycemia.
Interaction
Cationic Medicines: Cationic drugs are removed from the body by renal tubular secretion: Use at your own risk.
Phenprocoumon: Metformin may reduce phenprocoumon's anticoagulant impact. As a result, careful monitoring of the INR is advised.
Metformin's hypoglycemia impact can be reduced by taking levothyroxine. Blood glucose levels should be monitored, especially when thyroid hormone therapy is started or discontinued, and metformin dose should be modified if necessary.
Contraindications
This pill should not be used by anyone who has the following conditions:
Renal illness or dysfunction, as shown by serum creatinine levels of 1.5 mg/dL [males], 1.4 mg/dL [females] or aberrant creatinine clearance, can also be caused by diseases including circulatory collapse (shock), acute myocardial infarction, and septicemia.
Acute or chronic metabolic acidosis with or without coma, including diabetic ketoacidosis.
An allergic reaction to this pill or sitagliptin in the past, such as anaphylaxis or angioedema.
Because the use of such products might induce an abrupt change in renal function, this tablet should be temporarily stopped in patients having radiologic tests requiring intravascular injection of iodinated contrast materials.
Side Effects
Diarrhea, upper respiratory tract infection, and headache were the most prevalent adverse events recorded in 5% of patients who started sitagliptin and metformin at the same time, and were more common than in placebo individuals.
Hypoglycemia and headache were observed in 5% of patients treated with sitagliptin in combination with sulfonylurea and metformin, and were reported more often than in patients treated with placebo in combination with sulfonylurea and metformin.
In 5% of patients treated with sitagliptin in combination with insulin and metformin, hypoglycemia was the only adverse response observed, which was more prevalent than in individuals treated with placebo in combination with insulin and metformin.
Nasopharyngitis was the sole side effect observed in 5% of patients who received sitagliptin monotherapy, and it occurred more often than in individuals who received placebo.
Diarrhea, nausea/vomiting, fatulence, stomach pain, indigestion, asthenia, and headache are the most common (>5%) side effects associated with the start of metformin therapy.
Pregnancy & Lactation
Pregnancy Classification B. Because no appropriate and well-controlled trials with Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP or its individual components have been conducted in pregnant women, the safety of Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP in pregnant women is unknown. This pill should only be taken during pregnancy if absolutely necessary.
Sitagliptin is not known to be excreted in human milk. Because many medicines are excreted in human milk, this tablet should be used with caution if given to a nursing mother.
Precautions & Warnings
Lactic Acidosis-
Lactic acidosis can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic insufciency, renal impairment, and acute congestive heart failure.
Symptoms include malaise, myalgias, respiratory distress, increasing somnolence, and nonspecifc abdominal distress. Laboratory abnormalities include low pH, increased anion gap and elevated blood lactate.
If acidosis is suspected, discontinue this tablet and hospitalize the patient immediately.
Regular monitoring of thyroid-stimulating hormone (TSH) levels is recommended in patients with hypothyroidism.
Long-term treatment with metformin has been associated with a decrease in vitamin B12 serum levels which may cause peripheral neuropathy. Monitoring of the vitamin B12 level is recommended.
Others-
Do not use this tablet in patients with hepatic disease.
There have been postmarketing reports of acute renal failure, sometimes requiring dialysis. Before initiating this tablet and at least annually thereafter, assess renal function and verify as normal.
There have been postmarketing reports of acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. If pancreatitis is suspected, promptly discontinue this tablet.
Measure hematologic parameters annually.
Warn patients against excessive alcohol intake.
May need to discontinue this tablet and temporarily use insulin during periods of stress and decreased intake of fluids and food as may occur with fever, trauma, infection or surgery.
Promptly evaluate patients previously controlled on this tablet who develop laboratory abnormalities or clinical illness for evidence of ketoacidosis or lactic acidosis.
When used with an insulin secretagogue (e.g., sulfonylurea) or with insulin, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia.
There have been postmarketing reports of serious allergic and hypersensitivity reactions in patients treated with sitagliptin (one of the components of this tablet ), such as anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. In such cases, promptly stop this tablet, assess for other potential causes, and institute appropriate monitoring and treatment, and initiate alternative treatment for diabetes.
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP or any other anti-diabetic drug.
Storage Conditions
Store below 25°C, away from light, in a dry area. Keep medications out of the reach of youngsters in a secure location. Do not use beyond the expiration date. Only on a qualified physician's prescription can it be dispensed.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.